Decades of use with nearly four billion doses to humans preceded recent use with COVID patients. From the chapter ‘Ivermectin sends COVID to lockdown,’ in my book The Defeat Of COVID.
Ivermectin is on the World Health Organization (WHO) List of Essential Medicines and is approved by the US Food and Drug Administration (FDA). This well-tolerated but potent anti-parasitic medicine has been prescribed billions of times in its 36-year history against a wide range of parasites. It is a drug in the avermectin family, so named because those compounds are produced by the soil organism Streptomyces avermitilis. It has also been studied and used against a wide range of viruses especially over the last decade, and there is evidence of potent antiviral effects against Influenza A and over a dozen other viruses tested. [309]
In a meta-analysis of 63 studies of ivermectin versus COVID-19 in humans, 100% of these have shown positive results. Studies were from all continents except Antarctica. Considered individually, 29 of those studies were found to be statistically significant regarding use of ivermectin alone. Over the 63 studies in meta-analysis, pooled effects showed 69% improvement in early treatment, and prophylactic use showed 86% improvement. Of those studies in the meta-analysis that were peer-reviewed, overall improvement in early treatment was found to be 70% (64% in randomized controlled trials), and 86% of those in which ivermectin was used prophylactically showed improvement (84% in randomized controlled trials).
Mortality from COVID-19 over all time periods of delay in treatment was 76% improved over controls (69% in randomized controlled trials), whereas mortality was improved 84% in early treatment of COVID-19 (82% in randomized controlled trials). Forty studies were excluded from the meta-analysis for complicating factors or insufficient detail reported, and these also showed 100% positive results.
It is estimated that the likelihood of an ineffective treatment showing such positive results as the above results in the 63 studies in the meta-analysis to date is exceedingly small. That probability is estimated to be one in one trillion. [310] The overall results of the meta-analysis were not only found to be “overwhelmingly positive,” but also “very consistent, and very insensitive to potential selection criteria, effect extraction rules, and/or bias evaluation.” The data in the meta-analysis are as of date of this article, and are continually updated as new studies are reported.
The first clinical trial of ivermectin in COVID-19 patients was an observational study in four Florida hospitals from March to May 2020. Even in patients with severe pulmonary involvement, mortality was 38.8% in the treatment group vs 80.7% in controls, and this group showed the strongest mortality difference from controls, which raised the possibility of ivermectin also being available as a salvage or rescue treatment. [311]
In a randomized controlled trial, patients given ivermectin were 8 times more likely to be medically released than those in the placebo group. This was even though the average age and number of comorbidities were later found to be somewhat higher in the experimental group than in the control group. [312]
The African continent has had remarkably low incidence of COVID-19, particularly equatorial African countries. It may be helpful to look at African countries where ivermectin has been used commonly for decades against the onchocerciasis that it has been prescribed for, to observe population-wide effects. In this population comparison, risk of COVID-19 death was found to be 88.2% lower and morbidity 85.7% lower in 31 countries where onchocerciasis is endemic and ivermectin is commonly used than in 22 countries where neither is the case, even though the latter group of countries has a higher life expectancy, 66 years vs 61 years. [313]
Ivermectin, for all its power against viruses, is among the safest of medicines that are in long-term and widespread use. [314] There are no known serious drug-related adverse events. [315] Again, it is commonly taken by the populations of 31 African countries for effect against endemic parasites. Dosing has been given as a single annual dose of 150 mcg/kg against filariasis. There have been very few serious adverse events reported over more than 30 years of use. 37 of approximately 14,000 patients treated in Ghana had symptomatic posture hypotension, associated with fainting or sweating or tachycardia. These were treated with corticosteroids. [316] This Lancet study determined its safety in pregnant women, and the risk of fetal damage was not greater than in control women’s fetuses. [317]
However, despite this safety data going back 3 decades, the US FDA has alleged, “Any use of ivermectin for the prevention or treatment of COVID-19 should be avoided as its benefits and safety for these purposes have not been established.” The FDA offered no supporting evidence for their claim. [318] One concerning risk is that ivermectin is sold over the counter for veterinary use, and if people feel desperate to use it to ward off COVID-19, they might break off too large a piece from a large horse pill. For this reason, it is much better to consult a healthcare provider for ivermectin use and dosing. To further enhance safety, liposomal ivermectin carriers have been developed. When these were used against Dengue fever, cytotoxicity was reduced up to 5 times, absorption was faster and in vivo efficacy was improved. [319]
Despite the spectacular worldwide effect profile, of excellent effect against COVID-19, with 0.26% observed minor side effects, and its use across several continents, ivermectin is widely shunned and ignored in western Europe and in the US. Here is a brief synopsis of how that came to be.
Ivermectin was invented in Japan in 1975 as an anti-parasitic drug by Satoshi Omura, a Kitasato University professor emeritus, which earned Dr. Omura the Nobel Prize in Biochemistry. Ivermectin turned out to be quite effective against a broad spectrum of parasites. The drug was so effective in eliminating a range of parasitic infections, and at very low cost, about $0.10 US, that 3.7 billion doses have been delivered to much of the world’s population since its invention. [320]
A cell culture study in April 2020 showed a 5000 times reduction in SARS-CoV-2 from one dose over 48 hours, compared to control samples. [321] Several Latin American countries, Egypt and India soon began to use it for COVID-19, and then South Africa and several European countries as well. However, resistance remained strong in the US and western Europe, following the vocal disapproval of The World Health Organization (WHO), The US National Institutes of Health (NIH), the US Food and Drug Administration (FDA) and the European Medicine Agency (EMA). These agencies all expressed disapproval of ivermectin for use with COVID-19 patients. Even after more than 20 randomized controlled clinical trials showed promising effect without adverse reactions, many western countries have still not adopted its use.
Caly, Druce, et al illustrate the IMP inhibition as follows:
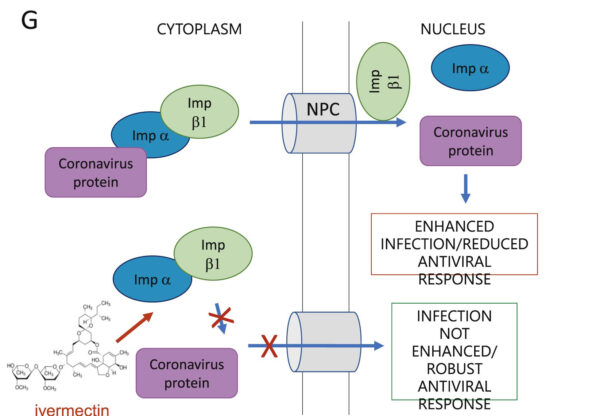
Figure © L Caly, J Druce, et al., Endnote 321
Social media companies censored ivermectin research. Even when the WHO commissioned and reported a meta-analysis of ivermectin, it was censored by YouTube. Only negative commentaries were permitted in western media. [322]
How does ivermectin send SARS-CoV-2 to lockdown? There are a number of mechanisms by which components of SARS-CoV-2 need to stay mobile and active in order to replicate, and thus to spread throughout the human body. It turns out that ivermectin binds several of these, which inactivates the virus. Let’s look at exactly what happens to bind or to lock down SARS-CoV-2.
RNA-dependent RNA-polymerase (RdRp) is one of the main enzymes used by SARS-CoV-2 to achieve RNA replication. It is required for viral genome replication, and therefore it is helpful if a nutrient or drug can act on it as an obstacle in some way. 173 drugs were tested in this study for their ability to bind RdRp (making it unavailable or inactive), including two examined in this book, hydroxychloroquine and vitamin C, although vitamin C was also found to have relatively high binding energy for RdRp in this study. Of all the drugs tested, ivermectin was found to bind RdRp with higher binding any energy than any other drug. [323]
One strategy against SARS-CoV-2, as well as other endemic and pandemic RNA viruses, has been to interfere with transport of viruses into a host cell’s nucleus. Ivermectin has been shown to accomplish this by binding, destabilizing and inhibiting the protein IMP alpha/beta1. When this protein is inhibited, viruses are unable to enter a cell’s nucleus, and therefore unable to replicate. Decreased infection results. IMP alpha/beta 1 has been inhibited in SARS-CoV-2 entry into nuclei by ivermectin. [324] Previously, it has been observed that ivermectin inhibited that same protein from entry of other RNA viruses, giving it a broad-spectrum antiviral effect. [325] [326] [327]
It turns out that ivermectin not only binds tightly to RdRp on SARSCoV-2, and IMP alpha/beta1; it also strongly binds the spike protein on SARS-CoV-2. This particular spike protein is trimeric, meaning it has 3 subunits which vary in amino acid sequences or other ways. It was observed that ivermectin binds all three of the SARS-CoV-2 subunits, both the structural S2 subunit, as well as both of the two functional S1 subunits. [328] This binding of all 3 subunits of the trimeric spike protein may be considered a trifecta of fortunate results of ivermectin in favor of the human host and in opposition to the SARS-CoV-2 virus.
Ivermectin has different mechanisms against parasites, already a miraculous healing drug for that use alone through much of the world’s population. However, now that we learn of its tremendous effect in binding both RdRp and all three trimers of the spike protein of SARS-CoV-2, we are certainly fortunate to have this medicine in our arsenal against COVID-19. It is inexpensive, and full COVID-19 treatment of an individual, from first dose till last needed can be less than one US dollar. Ivermectin is therefore available to even the poorest communities in the world. Ivermectin is being compared to the discovery of penicillin in its enormous impact, and perhaps was one of the greatest discoveries of the 20th century. [329] The fact that this tremendously effective, safe and low-cost antiviral drug is not as thoroughly known to the world as penicillin is a chasm of inexcusable and deadly ignorance that the COVID era is giving the world an opportunity to correct.
Reposted from the author’s Substack
References
309 K Sharun, K Dhama, et al. Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. May 30 2020. Ann Clin Microbiol Antimicrob. 19 (23). https://annclinmicrob.biomedcentral.com/articles/10.1186/s12941-020-00368-w
310 Covid Analysis. Ivermectin is effective for COVID-19: Real-time meta-analysis of 49 studies. Nov 26 2020. Updated Mar 31 2021. https://ivmmeta.com/
311 J Rajter, M Sherman, et al. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: The ivermectin in COVID nineteen study. Jan 2021. Chest. 159 (1). 85-92. https://www.sciencedirect.com/science/article/pii/S0012369220348984
312 R Chahla, L Ruiz, et al. Ivermectin repurposing for COVID-19 treatment of outpatients in mild stage in primary health care centers. Mar 30 2021. MedRxiv. https://www.medrxiv.org/content/10.1101/2021.03.29.21254554v1
313 H Tanioka, S Tanioka, et al. Why COVID-19 is not so spread in Africa: How does Ivermectin affect it? Mar 26 2021. MedRXiv. https://www.medrxiv.org/content/10.1101/2021.03.26.21254377v1.full-text
314 T Jabeen, M Khader, et al. A review on the anti-parasitic drug ivermectin for various viral infections and possibilities of using it for novel severe acute respiratory syndrome coronavirus 2: New hope to treat coronavirus disease – 2019. Jun 2020. Asian J Pharm Clin Res. https://www.researchgate.net/publication/343742900_A_REVIEW_ON_THE _ANTIPARASITIC_DRUG_IVERMECTIN_FOR_VARIOUS_VIRAL_INFECTIONS_AND_POSSIBILITIES_OF_USING_IT_FOR_NOVEL_SEVERE_ACUTE_RESPIRATORY_SYNDROME_CORONAVIRUS_2_NEW_HOPE_TO_TREAT_CORONAVIRUS_DI SEASE
315 J Sanz-Navarro, C Feal, et al. Treatment of human scabies with oral ivermectin. Sep 2017. Actas Dermos. 108 (7). 643-649. https://pubmed.ncbi.nlm.nih.gov/28385424/
316 J Remme, R Baker, et al. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. I: Effect on the microfilarial reservoir and the transmission of Onchocerca volvulus. Sep 1989. Trop Med Parasitol. 40 (3). 367-374. https://pubmed.ncbi.nlm.nih.gov/2617046/
317 M Pacqué, B Muñoz, et al. Pregnancy outcome after inadvertent ivermectin treatment during community-based distribution. Dec 15 1990. Lancet. 336 (8729). 1486-1489. https://pubmed.ncbi.nlm.nih.gov/1979100/
318 US FDA. FAQ: COVID-19 and ivermectin intended for animals. Dec 16 2020. https://www.fda.gov/animal-veterinary/product-safetyinformation/faq-covid-19-and-ivermectin-intended-animals
319 R Croci, E Bottaro, et al. Liposomal systems as nanocarriers for the antiviral agent ivermectin. May 8 2016. Int J Biomater. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4875998/
320 M Yagisawa, P Foster, et al. Global trends in clinical studies of ivermectin in COVID-19. Mar 10 2021. Japanese J Antibiotics. 74 (1). 44-95. http://jjacontents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf
321 L Caly, J Druce, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Jun 2020. Antiviral Res. https://www.sciencedirect.com/science/article/pii/S0166354220302011?via%3Dihub
322 M Turkia. A timeline of ivermectin-related events in the COVID-19 pandemic. Mar 2021. https://www.researchgate.net/publication/350496335_A_Timeline_of_Iver mectin-Related_Events_in_the_COVID-19_Pandemic
323 I Udofia, K Gbayo, et al. In silico studies of selected multi-drug targeting against 3CLpro and nsp12 RNA-dependent RNA-polymerase proteins of SARS-CoV-2 and SARS-CoV. Mar 25 2021. Network Mod Anal Health Inf Bioinf. 10 (22). https://link.springer.com/article/10.1007/s13721-02100299-2
324 L Caly, J Druce, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Jun 2020. Antiviral Res. https://www.sciencedirect.com/science/article/pii/S0166354220302011?via%3Dihub
325 M Tay, J Fraser, et al. Nuclear localization of dengue (DENV) 1-4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor ivermectin. Sep 2013. Antivir Res. 99 (3). 301-306. https://www.sciencedirect.com/science/article/abs/pii/S0166354213001599
326 K Wagstaff, H Sivakumaran, et al. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. May 1 2012. J Biochem. 443 (3). 851-856. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3327999/
327 S Yang, S Atkinson, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin alpha/beta1 heterodimer. May 2020. Antivir Res. 104760. https://www.sciencedirect.com/science/article/abs/pii/S0166354219307211
328 A Choudhury, N Das, et al. Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach. Future Vir. Mar 25 2021. https://www.futuremedicine.com/doi/10.2217/fvl-2020-0342
329 M Yagisawa, P Foster, et al. Global trends in clinical studies of ivermectin in COVID-19. Mar 10 2021. Japanese J Antibiotics. 74 (1). 44-95. http://jjacontents.wdc-jp.com/pdf/JJA74/74-1-open/74-1_44-95.pdf


